We have briefly mentioned the structure of the atom during previous classes. Today we will attempt to understand this structure more fully, including how atoms emit electromagnetic energy ("light"), and how astronomers use that light to unravel the nature of distant objects. First we return to the basic structure of the atom. All of the elements in nature are composed of just three types of subatomic particles: protons, electrons and neutrons. Protons and neutrons stick together to form the nuclei of atoms (nuclei is the plural of nucleus). And the electrons orbit around the nucleus sort of like planets orbiting the Sun.
Electrons have a negative charge while protons have a positive charge. The attraction between the negative charge of an electron, and the postive charge of the proton, is what keeps the electrons in orbit around the nucleus. Instead of gravity, it is the "electrostatic" charge attraction that holds atoms together. It is informative to look at the masses of these two particles. A proton has a mass of 1.6 X 10-24 gm, while the electron has a mass of 9.1 X 10-28 gm. Electrons have a much smaller mass than the proton--this is why they are the particle in orbit--like the planets, the electrons orbit the nucleus because they are the less massive objects. Because of the size of these tiny numbers, physicists use the mass of the proton as the unit when talking about the masses of atoms. In this system, the proton has a mass of "1", and an electric charge of +1 unit, while the electron only has a mass of 1/1836 proton masses (that is a proton is 1,836 times more massive than an electron), and a negative charge of -1 unit.
Neutrons are particles with the same masses as protons, but they do not have any charge--we say they have a "neutral charge", or a charge of "0". Thus, neutrons do not really affect the chemistry of atoms, though they play a significant role in radioactivity. You might think that neutrons are one of those peculiar mistakes of nature, but there is a very good reason for why they exist. If you are interested, you can go to this excellent site to read more about the structure of the atom, the nucleus, and what protons, neutrons and electrons are, and how they interact.
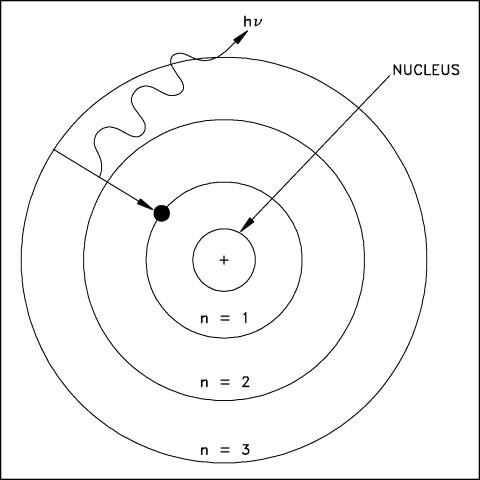
A simple way to visualize the structure of the atom is the solar system model, where we have electrons (planets) orbiting the nucleus (the Sun) in particular orbits. A schematic of this layout for the hydrogen atom is shown below:

In an atom, there are specific "orbits" (n=1, n=2, etc.) in which an electron can actually orbit the nucleus. The rules for determining these orbits are quite complicated, and we will not discuss them here (it is the physics of "quantum mechanics" that is needed to fully describe the structure of atoms), but we say that the orbits are "quantized". An electron revolves around the nucleus in a particular orbit. There are a set of orbits in every atom determined by how many protons are in the nucleus, and how many other electrons are in the atom. In the hydrogen atom there is one electron in orbit around the one proton that comprises the hydrogen atom nucleus. As we discussed last class, there can be other types of hydrogen. In the hydrogen isotope deuterium, the proton is joined by a neutron. In the hydrogen isotope tritium, there are two neutrons and one proton in the hydrogen nucleus. Note, however, that the number of neutrons has no effect on the charge of the nucleus, so all of the isotopes of hydrogen act the same way--it is the number of protons and electrons that determine the chemical properties of an atom (giving rise to the periodic table). Thus, water ("H2O") can be made with any of the isotopes of hydrogen and it will still act just like water! Figure 4.7 of the textbook summarizes the terminology of of atoms, here is the part dealing with isotopes:
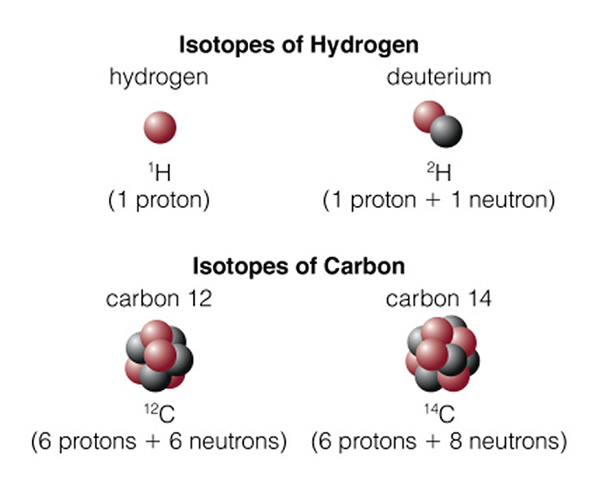
On the top we have normal hydrogen (left) with one proton, and deuterium (right) with a proton and neutron. Larger/heavier atoms like carbon (in the bottom of the figure), have multiple isotopes. Normal carbon is "carbon 12" (12C), while the unstable, radioactive isotope of carbon is "carbon 14" (14C). Normal carbon, 12C, is called that because the mass of the carbon atom is 12 units of proton mass. There are six protons and six neutrons in the carbon nucleus. But the atomic number of carbon is "six", as it is the number of protons (which is the same as the number of electrons, giving rise to the ordering of the elements in the periodic table) in an atom that determines how it will behave:
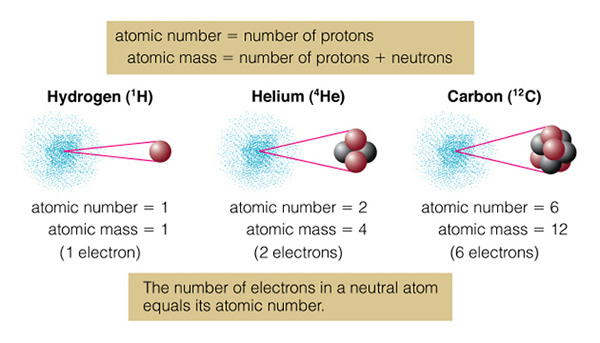
Normally, all atoms are "neutral" (at least on the Earth), that is there are exactly the same number of protons and electrons in the atom. If, however, you remove one of the electrons from the atom, you form an "ion". For example, if carbon normally has six electrons and six protons, it has a neutral charge--it is uncharged (charge of "0"), and we could write this as C0. If we remove one electron from the carbon atom, the nucleus will still have six protons, but now there are only five electrons orbiting the nucleus. This carbon atom now has a charge of +1 (since we took away a -1 charge). We call this atom "a singly charged ion of carbon", and physicists write it as C+1. Astronomers have their own notation for this. Astronomers call neutral carbon "C I" instead of C0, and C+1 as "C II". This helps eliminate having to put in lots of superscripts! What do you think C III means? That's right, it is carbon with two electrons removed (C+2).
Let's return to talking about electrons and orbits. Each of the orbits in an atom corresponds to a certain amount of energy. Electrons with lots of energy are in orbits far away from the nucleus, while electrons with low energy are close-in to the nucleus. If we put a bunch of hydrogen atoms inside a metal box, and chill this box in a very cold freezer, we will find that the electrons in all of the hydrogen atoms will be in the lowest energy orbit. Physicists call this the "ground state". Electrons prefer to orbit close-in to the nucleus--they like the smallest orbit possible. If we now shine a high intensity light on our hydrogen atoms, we will find that some of the electrons move out to larger orbits. What has happened? Well, some of the electrons have absorbed some of the light energy, and by increasing their energy, they have to move out to a more distant orbit. To get back to the ground state, the electron will have to give-up this added energy. How does it do this? By emitting light! In nature, this process is happening all of the time, electrons inside atoms absorb some light, move out to a higher orbit, and, a little while later, turn around and emit that light. Electrons are not very good at holding on to energy--in fact, in the hydrogen atom, the electron will usually only hold onto this energy for a tiny fraction of a second before giving it back. Electrons like to be in the ground state, and not wander too far from home.
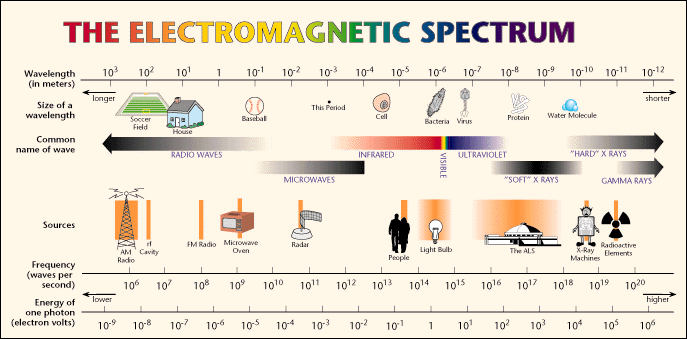
What makes this process special is the quantized nature of the electron orbits. As we said, there are only a special set of allowed orbits for an electron. Thus, there are only a special allowed sets of energy for an electron. Each orbit has a particular energy associated with it--we say the energy of an orbit is "quantized". To move from one orbit outwards to another, the electron must absorb exactly the right amount of energy--it cannot absorb more or less, it has to be exactly right (sort of like Goldilocks and the porridge!). The electron must absorb a photon of light with exactly the right amount of energy. Oh, oh, a new word: photon. What is a photon? We have already encountered the electromagnetic spectrum--it runs from low energy forms of light like radiowaves and microwaves, through the infrared and visible (where our eyes work), to higher forms of energy like ultraviolet, X-rays and Gamma-rays. Here is the plot of the electromagnetic spectrum:
Light is an electromagnetic wave--a small packet of energy that travels through space (and air and glass) as a wave, but it carries energy in a packet we call a "photon", and when a light wave encounters matter, it acts like a particle--the photon--and this particle can collide with electrons. It is a difficult concept to understand. When light is traveling through space, it acts like a wave (just like water waves), and it has a "wavelength":
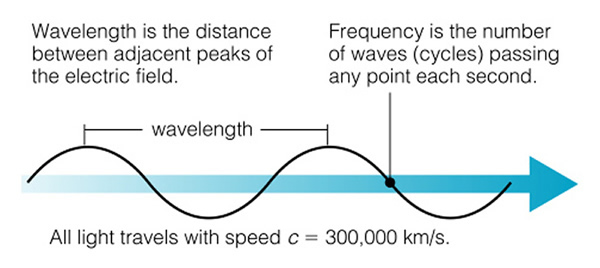
Light (and that includes all kinds of light from radio to gamma-rays) is a wave, and we classify light by its wavelength (Fig. 6.5). The shorter the wavelength, the more energy that type of light has. The longer the wavelength, the less energy the light has (note that the two figures about the electromagnetic spectrum presented here have the direction of high and low energy reversed. Astronomers always put low energy to the right, and high energy to the left--just an oldtime convention, as shown in the next figure 6.5):
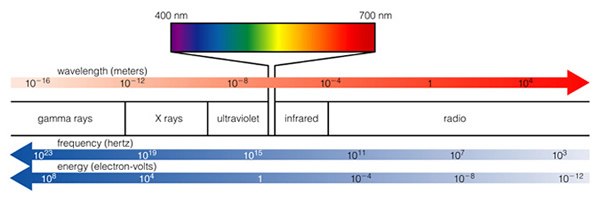
Our eyes are sensitive to visible light, because that is the dominant kind of light emitted by the Sun. Evolution has insured this--it would be hard to see anything if our eyes were sensitive to X-rays, as X-rays from the Sun do not penetrate our atmosphere, and are not easily reflected! Thus, we can't use X-rays, and thus no animals have eyes that are sensitive to something besides visible light (note that some types of snakes have special organs that are sensitive to infrared light--heat radiation--and this allows them to hunt in the dark by detecting the heat radiated by a mouse, while some insects and animals have eyes sensitive to the bluest visible light, called "ultraviolet"). What you need to grasp from this discussion is that light carries energy, and electrons can absorb light, and electrons can emit light. They do this by absorbing or emitting a photon with a certain amount of energy. Here is our model of the atom again (the "Bohr" model, after Niels Bohr):

This diagram shows an electron in a hydrogen atom moving from the n=3 orbit to the n=1 (ground state) orbit by emitting a photon (the squiggly arrow). This photon will have a prescribed (and predictable!) amount of energy. The electron could move back out to the n=3 from the n=1 orbit by absorbing a photon with exactly the same amount of energy as the one it just emitted. Because the energy of the orbits are quantized, the photons emitted by the electrons can only have certain values of energy. This is shown here, a more complicated diagram of the hydrogen atom:
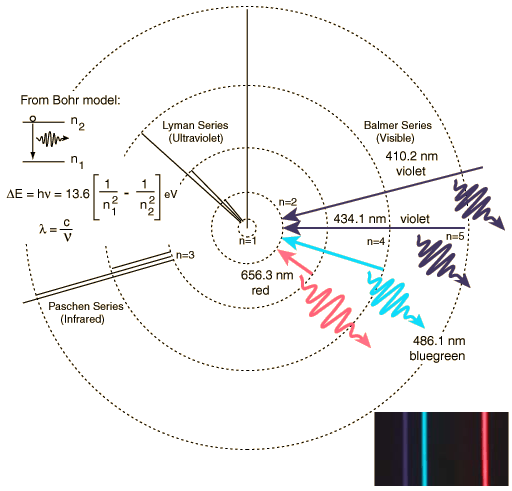
This diagram now shows several "transitions" (that is the movement of electrons between orbits). If an electron moves from the n=3 orbit to the n=2 orbit it emits red photons. If the electron moves from the n=4 to the n=2 orbit, it emits a blue photon. The further out the orbit, the more energy it has, so when it transitions inwards, it releases photons with more energy, and more energy means bluer, and bluer, until you reach the ultraviolet. A bigger transition releases more energy, and more energy means shorter wavelength. We can look at these transitions a different way, instead of thinking of them as circular orbits, we can think of them as being steps. The first few steps are big, but as you go further out in the atom, the steps become smaller and smaller. The following diagram (Fig. 6.8a) is this alternative way to view electronic transitions:
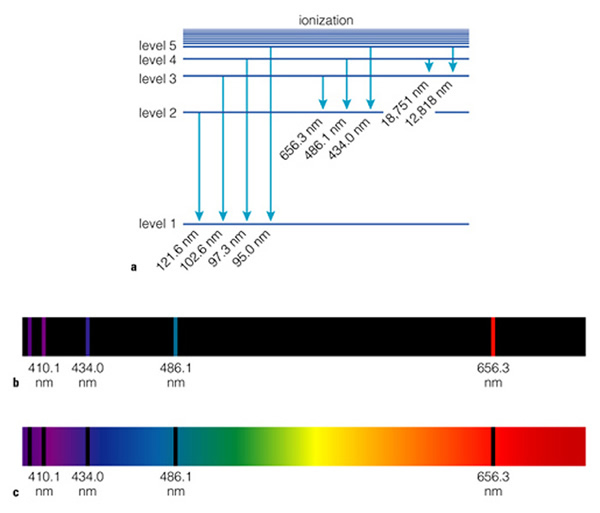
Note that jumping from a higher to a lower "level" (level=orbit!) in the atom releases energy. Jumping from a lower to a higher level requires energy. Thus, we have two kinds of "spectra", emission line spectra (Fig. 6.8b), and absorption spectra (Fig. 6.8c). Note that the "emission lines" are at the same place as the "absorption lines"--they have to be, as the photons that are emitted have identical wavelengths as those that are absorbed. So why are we spending so much time on this? Because each element has a different internal structure, and because of this, the spectrum of each element is unique. Here are three examples (Fig. 6.8):

Just like using fingerprints to identify people, astronomers can use spectra to identify elements! This is the most important tool that astronomers have. By knowing how much energy it takes to create certain spectral lines, we can figure out the temperature of the gas that is emitting this light. Thus, we are also able to measure the temperature of gas using spectral lines. We will learn a bit later that we can use spectra to find out how fast an object is moving. Let's look at the spectrum of the Sun:
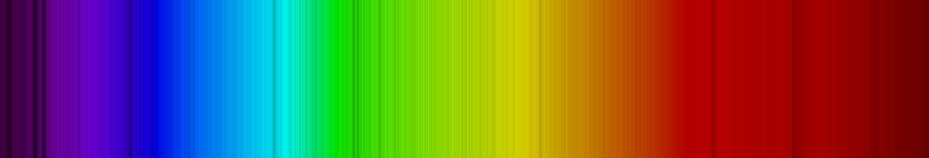
Look at all of those lines! It takes quite a bit of detective work to fingerprint the Sun, and identify all of those lines (but it has been done). You see that the spectrum of the Sun is all absorption lines. Why is that? Well this takes just a bit more explanation, and the relevant diagram is Figure 6.14:
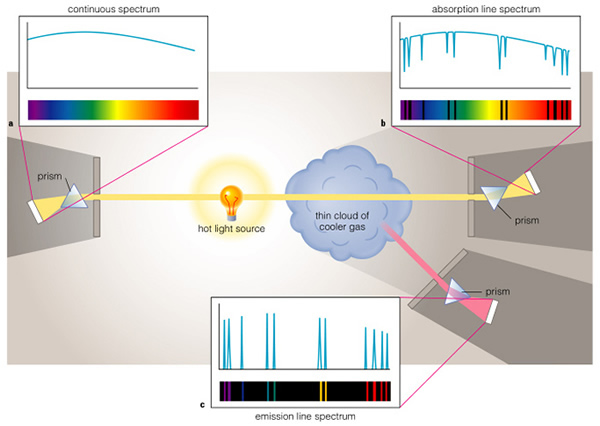
There are three kinds of spectra: absorption, emission, and "continuous" ("spectra" is the plural form of the word "spectrum"). We have encountered the first two already--we need to discuss the continuous spectrum. In hot, dense objects (like inside the Sun, or in the metal filament of a light bulb), some electrons are free to move around. If some electrons are free, however, that means that some of the atoms in the Sun, and in the filament of the light bulb, have positive charges, as they have lost electrons. As these electrons pass by a charged atom (an ion), they can become temporarily stuck in an orbit around that atom, jump between levels and emit photons. But in the meantime, another electron comes zooming by and runs into this electron--it can knock it out of the orbit, and this one now gets trapped in this atom (sort of like musical chairs!). All of this jumping around by the electrons means that there are always a bunch of electrons emitting light. The question you should be asking is "Why isn't the spectrum of a light bulb or the Sun just a bunch of lines?" This is because the nearby ionized atoms can alter the orbits of some of these electrons, and by doing so slightly change the energy levels of those atoms. Because there are billions and billions of nearby atoms, we have billions, and billions of possible orbits. This means that just about every wavelength of light is emitted by a hot, dense object! I know this is complicated, but this is the explanation for why a light bulb emits the full spectrum of visible light, as does the Sun.
But why an absorption spectrum for the Sun? Because, above the hot, dense parts of the Sun, is a cooler, less-dense atmosphere. In this atmosphere, the atoms act normally and can absorb and emit light in the normal fashion. As Figure 6.14 shows, we see an aborption line spectrum because we see a hot continuum source behind the thin, cool atmosphere; we see the lines in absorption. Cool gas absorbs energy.
The only time we see an emission line spectrum is when the gas is very hot, and has a low density. If the gas has a high density, the orbits are distorted and we see a continuum spectrum. But in low density gas, the atoms are further apart, and they cannot change the orbits of electrons in other atoms. In this case we see an emission line spectrum. You will have a lab exercise where you look at the spectra of atoms later this semester.